All sectors of biotech - diagnostics, therapeutics, medical devices, and biofuels and biomaterials – use vast amounts of data and documents, much of it is confidential and proprietary. The industry has an ongoing need to store these materials securely and to share them efficiently. Traditionally, file cabinets and on-site servers met a biotech company’s storage needs.
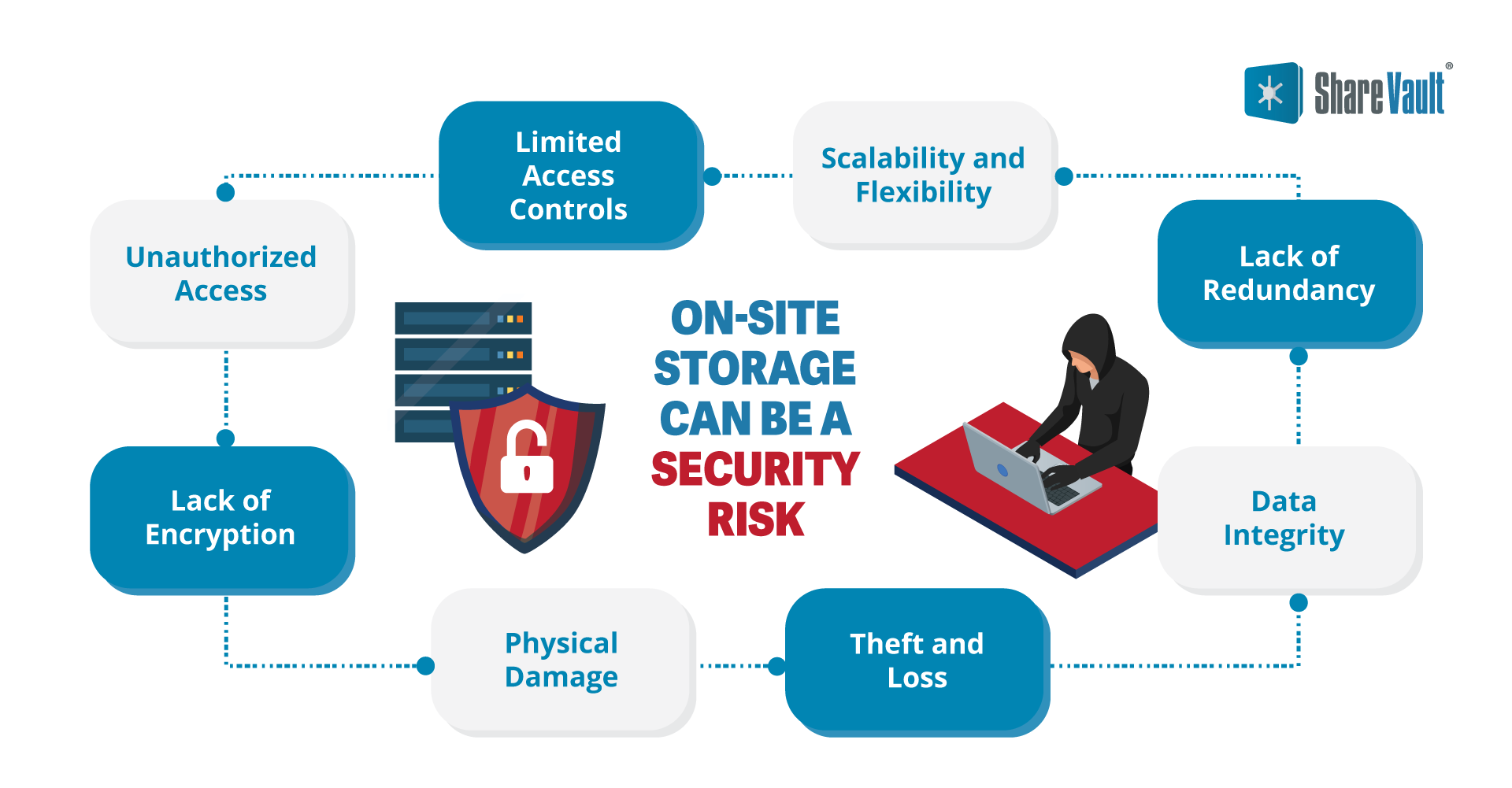
On-site Storage Can Be a Security Risk
- Limited Access Controls: A biotech firm should have strict security protocols for accessing archived files, with permissioning safeguards not only to the server or file storage area but also to individual folders, files, and documents. Depending on the physical layout of the storage area, enforcing these protocols can be challenging, with an increased risk of unauthorized individuals gaining access to sensitive information.
- Unauthorized Access: A physical storage system can be vulnerable to unauthorized access by individuals, due simply to poor staff security protocols or by theft.
- Lack of Encryption: Physical storage data and files do not benefit from encryption, which would provide protection from unauthorized access, even in the case of theft.
- Physical Damage: Physical storage devices are susceptible to damage from fire, flooding, power surges, or mishandling, resulting in document and data corruption or loss.
- Theft and Loss: Data files or documents in a physical storage area can be stolen. Once in the hands of criminals, confidential files can lead to ransom demands and legal exposure for the biotech firm.
- Data Integrity: Physical storage media can experience data corruption over time due to irregularities in office environment controls or manufacturing defects. This can result in unreadable or altered or unreadable data.
- Lack of Redundancy: Physical storage systems may not have adequate redundancy of critical files. Without backup copies, data recovery becomes difficult, if not impossible.
- Scalability and Flexibility: Expanding capacity of a physical storage area can be challenging and costly, which can limit the scalability of data storage.
Secure File Sharing: A Virtual Data Room is a Better Way to Store Confidential Data Files and Documents
Biotech companies already use online storage in product development and for other daily business functions, making use of apps like DropBox, Box, Google Drive, and Microsoft SharePoint & OneDrive. While the security of these apps is sufficient for many daily tasks, they lack the enterprise-grade security necessary for confidential documents. The better solution is a virtual data room (VDR).
A VDR is a secure online environment for storing and sharing data files and documents, with robust, enterprise-grade security features that control who has access to the protected environment and what actions a user can take with a document or data file. Since the data is stored in the cloud, files are not subject to physical harm. Authorized users can access folders and files at any time, from anywhere in the world.
Among VDR providers, ShareVault is the smart choice for biotech companies. ShareVault has been serving biotech and life science companies for more than 15 years, for well-known companies as well as startups. A ShareVault data room is 21 CFR Part 11 Compliant, and every document and data file is automatically encrypted on upload to the VDR. With its advanced security and helpful features, ShareVault is the preferred Business Solutions Program provider of the Biotechnology Innovation Organization (BIO) and more than 50 other life science trade organizations.
ShareVault also includes built-in tools to streamline essential processes, like product development program management and regulatory submissions.
How to Transition from a Physical to a Virtual Data Room
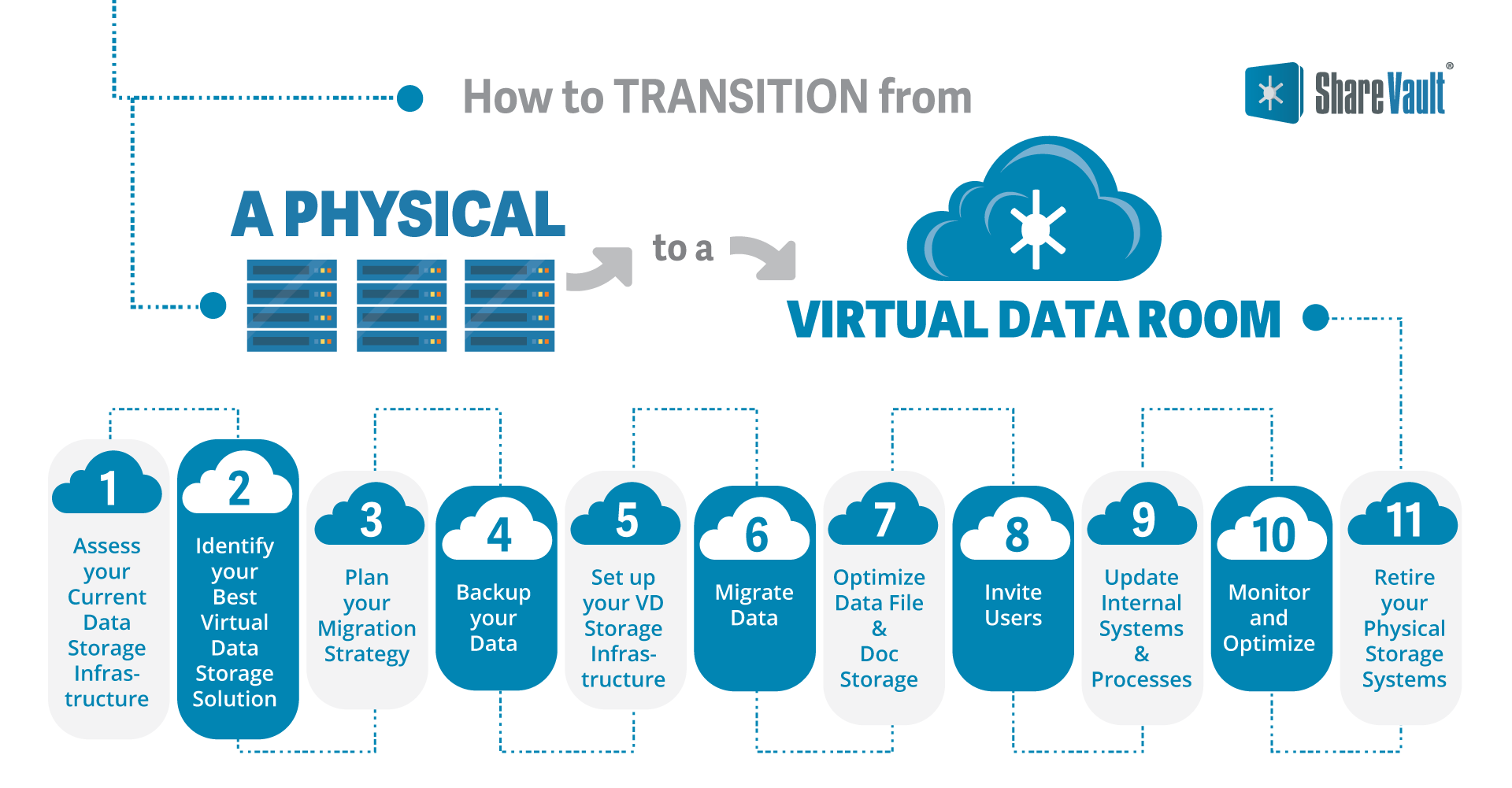
1. Assess your Current Data Storage Infrastructure
Evaluate your existing physical data storage systems including servers, hard drives, and other storage devices. Understand the capacity, performance, and limitations of your current setup.
2. Identify your Best Virtual Data Storage Solution
Research and identify virtual storage solutions that align with your organization's needs.
3. Plan your Migration Strategy
Outline the steps, timelines, and resources required for the transition. Consider factors such as data security, downtime, data integrity, and compatibility with existing systems, and how the transition will impact ongoing projects.
4. Backup your Data
Before transitioning, ensure you have proper backups of all data files and documents as a safety net in case of any unforeseen issues during migration.
5. Setup your Virtual Data Storage Infrastructure
Set up a cloud storage account. ShareVault will designate a support team that has experience in biotech that will assist in the transition. These pros will walk you through the process of setting up folders, uploading files, and authorizing team member access. Since most biotech companies making a move to a VDR are anxious to get the transition done in the least amount of time, ShareVault support is available 24/7/365.
6. Migrate Data
Transfer your data from your physical storage systems to the virtual data storage solution. With ShareVault, transferring electronic data files is a simple drag-and-drop upload. Those transfers are protected by ShareVault encryption—files in transit are via HTTPS over Secure Sockets Layer (SSL), which maintains AES-256 encryption in transit. Additional ShareVault features like bulk upload and integration with file sharing apps speed the process.
7. Optimize Data File and Document Storage
ShareVault has built-in tools to expedite the transition to virtual storage, including the hierarchical tag, which is a hashtag notation that allows a document to appear in multiple folders, without the need to duplicate files and manually seed them.
8. Invite Users
The biotech company’s designated VDR administrator invites participants, who then generate passwords. The IP address of each user device is registered. Each user has permissions specific to each document and data file, such as read only, copy/paste, print, and/or download. Since participants can change over time, the VDR administrator can alter or suspend authorization at any time.
9. Update Internal Systems and Processes
Adjust the company’s IT systems, applications, and processes to adapt to the new virtual storage environment. This may include reconfiguring backup and recovery procedures, modifying data management policies, and training employees on optimal use of the virtual data room and ShareVault’s software features.
10. Monitor and Optimize
Monitor your team’s use of the VDR. Consult with your ShareVault support rep to answer questions and to optimize user performance and efficiency.
11. Retire your Physical Storage Systems
Once you have completed a successful migration to the virtual data room, decommission and retire unneeded physical storage systems.
ShareVault Improves the Regulatory Process
When regulatory submissions are required, ShareVault’s enterprise-grade secure VDR is the preferred platform for document submissions: Electronic Trial Master Files (ETMF), Investigational New Drug (IND) applications, New Drug Applications (NDA), Abbreviated New Drug Applications (ANDA), Biologics License Applications (BLA), Drug Master Files (DMF), Biologics Master Files (BMF) Emergency Use Authorizations (EUA), and other regulatory submissions.
ShareVault’s built-in software tools include file monitoring — a detailed history of use for every document and data file. When regulators perform an audit, the company can produce a record with a single click.
Should regulators require access to documents, the biotech company’s VDR administrator can provide access via the same permissioning protocols as any other user.
ShareVault: The Best VDR Solution for Biotech
ShareVault’s virtual data room provides the enterprise-grade protection for all data files and documents that contain confidential and proprietary information, and its built-in software assists the transition from physical to virtual data storage. A ShareVault representative will fill in the details and provide a customized pricing structure for VDR use.
To learn more, contact ShareVault today!
