The Promise and Challenges of Antibody-Drug Conjugates (ADC)
24 January, 2020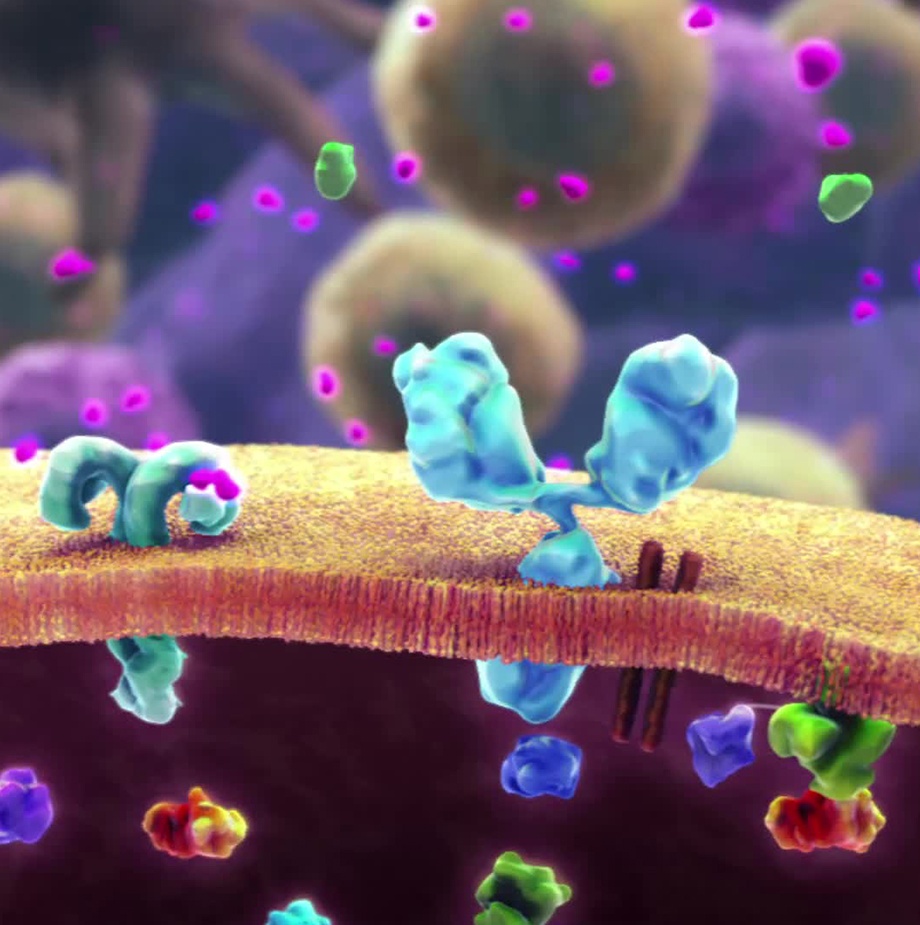
Antibody-drug conjugates, or ADCs, are a new class of highly potent biopharmaceutical drug that can ferry drugs into cancer cells while minimizing or eliminating damage to healthy tissue. This next generation of “weaponized” antibody therapies combine the unique targeting capabilities of antibodies, which can discriminate between healthy tissue and cancer tissue, with the powerful cell-killing ability of cytotoxic drugs. By linking monoclonal antibodies, via a chemical linker, to a biologically active drug or cytotoxic compound, scientists are optimizing the features of both components.
ADCs combine the unique, highly specific, properties and anti-tumor activity of monoclonal antibodies (mAbs) that are tumor-specific, but not sufficiently cytotoxic, with the potent cell-killing activity of cytotoxic small molecule drugs that are too toxic to be used on their own.
To date, six ADCs have received market approval and over 100 are in clinical development. Sales of ADCs are expected to reach $2 billion in 2018 and grow to $12 billion by 2024 (GlobalData).
Simply put, antibody-drug conjugates deliver “deactivated” cytotoxins to specific cancer cells. Once in the tumor cell, the cytotoxin is released and regains its full, cancer-killing, cytotoxic power. This, in turn, leads to rapid cancer cell death.
 While the concept of ADCs is relatively straightforward and easy to understand, the design and synthesis of a fully functional and effective antibody-drug conjugate is remarkably challenging.
While the concept of ADCs is relatively straightforward and easy to understand, the design and synthesis of a fully functional and effective antibody-drug conjugate is remarkably challenging.
On January 24, 2019, ShareVault, in partnership with Pullan Consulting, Cello Health and BIO hosted a web panel discussion exploring the mushrooming field of ADCs and what it means for patient care, licensing deals, and the oncology marketplace.
During this 90-minute web panel discussion, moderated by Linda Pullan, PhD, of Pullan Consulting, the panelists discussed a broad range of topics, including:
- What is needed for an ADC target?
- What kind of drugs are suitable for attachment to an antibody?
- What indications are being pursued?
- How are advances in linker technology improving the delivery of cytotoxic agents to cancer cells?
- Can ADCs have applications beyond that of tumor killing?
- What are some of the challenges of manufacturing ADCs, and how are those challenges being overcome?
- How are drug companies dealing with the complexities of internalization and the high cost of goods?
- What kind of data will be required to drive ADC licensing deals?
- What is the future of ADCs?
- And much more
If you missed this informative web panel discussion exploring the exciting field of ADCs and what it means for patients, caregivers, investors and licensing deals, you can request the recording below.
