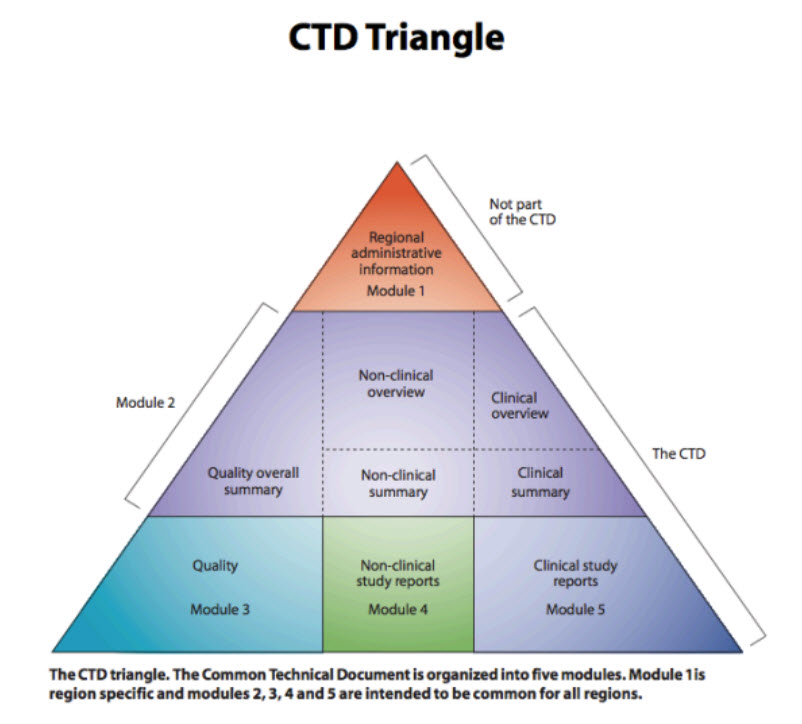
CTD is an acronym for “Common Technical Document,” which is the pharmaceutical industry’s internationally agreed upon format for the preparation of applications regarding new drugs intended to be submitted to the Federal Drug Administration (FDA) and other regulatory authorities in participating countries. The eCTD (electronic Common Technical Document) is the electronic version of this standard.
Beginning on May 5, 2017, companies must use the eCTD format to submit Investigational New Drug Applications (INDs) New Drug Applications (NDAs), Abbreviated New Drug Applications (ANDAs), Biologics License Applications (BLAs) and Master Files. Submissions of Commercial INDs to the FDA must be made in eCTD format beginning May 5, 2018. After those deadlines, the FDA will not receive submissions that do not adhere to the requirements stated in the eCTD Guidance.
In the U.S., the FDA Electronic Submissions Gateway (ESG) is the central transmission point for sending information electronically to the FDA. Within that context, the FDA ESG is a conduit along which submissions travel to reach their final destination. It does not open or review submissions, but instead automatically routes them to the proper FDA Center or Office.
WHAT IS AN eCTD VIEWER?
Because the eCTD format is a complex nested hierarchy that is not easy to read, several software companies have developed eCTD viewers, which make reviewing regulatory documents faster and more convenient. These viewers also make it easier and more efficient to identify the critical information contained in the submission.
WHY DO ORGANIZATIONS USE eCTD VIEWERS?
eCTD viewers were designed with ease-of-use in mind, enabling the complex XMLstructure of an eCTD lifecycle to be presented in a user-friendly manner. eCTD viewers reduce review times, increase response times to Agency requests and ensure faster approvals for new products.
HOW DO eCTD VIEWERS WORK?
eCTD viewers can load and process any eCTD submission, as long as sequences conform to the International Conference on Harmonisation (ICH) and regional eCTD specifications. An eCTD viewer's fluent user interface normally includes a filter pane feature, a backbone tree (including current, cumulative and custom views) and a set of tabbed panes to quickly preview envelopes (regional information), metadata attributes, documents, lifecycle info, STFs, PDF hyperlinks, and other details. It is easy to configure the viewer to address specific customer needs.
WHAT TO LOOK FOR IN AN eCTD VIEWER
- Ease of Use
Users shouldn’t have to learn a new language to use an eCTD viewer. eCTD viewers have been designed for the sole purpose of viewing eCTD submissions, specifically for users who do not need to know the technical aspects of the eCTD. Users should be able to start using an eCTD viewer without any training. - Speed
eCTD viewers should not be clunky, cumbersome, slow or frustrating. Look for an eCTD viewer that outperforms the competition in terms of loading speed and the time it takes to construct the full lifecycle current and cumulative views. - Compliance
The eCTD viewer you choose ought to be compliant with the latest eCTD specifications as well as be backwards-compatible with previous specifications from ICH and Canadian, E.U., Japanese, Swiss, Taiwanese and U.S. regions. You should be able to view any eCTD submissions, even those produced by other software vendors’ publishing tools, through your eCTD viewer, as long as they conform to ICH and regional eCTD specifications. - Easy Navigation and Viewing
Your eCTD viewer should have features that ensure fast and easy navigation throughout the viewer, such as a table of contents that is compliant with the latest eCTD and regional section headings. It should employ a multi-tab feature, just like a browser, enabling the user to keep multiple documents open and organized. The eCTD viewer should also be able to filter modules and have powerful and fast search functionality, making it easy to find submission metadata and document titles. - Lifecycle Operations
A good eCTD viewer should have an intuitive visual indication for lifecycle operations and status, and it should provide easy access to lifecycle history. Users should be able to open all “appended” documents with one click, and it should support both implicit and explicit lifecycle operation models. - Productivity Tools
Look for other features in an eCTD viewer designed to increase productivity, such as the ability to open a specific submission or the entire application, smart caching (compression and refreshing techniques that dramatically cut down on loading time), batch printing and the ability to download documents from the server for convenient off-line viewing.
WHAT IS THE CTD STRUCTURE?